21 CFR Part 11 Compliance
|
|

Data integrity, security, and traceability are paramount in the pharmaceutical and biotechnology industries. The US Food and Drug Administration (FDA) introduced 21 CFR (Code of Federal Regulations) Part 11 to establish electronic records and signature criteria. This regulation outlines the requirements for using electronic records and signatures instead of traditional paper-based systems in FDA-regulated industries.
Understanding and implementing 21 CFR Part 11 compliance can be challenging, but it is essential for organizations that wish to maintain regulatory approval and avoid potential legal consequences.
This article provides an overview of 21 CFR Part 11 compliance and offers guidance for navigating the related complex requirements and their automated testing.
Overview of 21 CFR Part 11
21 CFR Part 11, or Title 21 of the Code of Federal Regulations, Part 11, establishes criteria for electronic records and signatures to be considered trustworthy, reliable, and equivalent to paper records and handwritten signatures. The regulation applies to any organization that submits information to the FDA in electronic form. Or if they wish to maintain electronic records in lieu of paper records.
The regulation is divided into three main sections:
Subpart A – General Provisions
Subpart A outlines the scope and application of the regulation, defining key terms and establishing the foundational guidelines that apply to electronic records and electronic signatures within the context of FDA regulations.
11.1 Scope: Specifies the types of submissions that are covered by the regulation, mainly those related to FDA-regulated products and activities.
11.2 Implementation: Discusses the conditions under which electronic records and electronic signatures are considered equivalent to paper records and handwritten signatures.
11.3 Definitions: Provides definitions for terms such as “biometrics,” “closed system,” “open system,” “digital signature,” and others. These definitions are crucial for understanding the requirements and ensuring compliance.
Subpart B – Electronic Records
Subpart B sets forth requirements that ensure the integrity, reliability, and, if applicable, confidentiality of electronic records.
11.10 Controls for Closed Systems: Lists controls required to ensure the integrity of electronic records, including system validation, the ability to generate accurate and complete copies of records, protection of records, and audit trail requirements.
11.30 Controls for Open Systems: Similar to 11.10, it adds the need for additional measures to ensure the integrity and confidentiality of records accessed or stored in systems exposed to environments beyond controlled access.
11.50 Signature Manifestations: Specifies that signed electronic records must clearly indicate the printed name of the signer, the date and time when the signature was executed. Also, the associated meaning (such as review, approval, responsibility) with the signature.
11.70 Signature/Record Linking: Electronic signatures and records must be linked in such a way that the signature cannot be excised, copied, or otherwise transferred to falsify an electronic record by ordinary means.
Subpart C – Electronic Signatures
Subpart C details requirements related to electronic signatures, including their execution and attribution.
11.100 General Requirements: Electronic signatures must be unique to individuals and must not be reused or reassigned to anyone else.
11.200 Electronic Signature Components and Controls: This section delineates the requirements for electronic signature components, such as the use of identification codes and passwords.
11.300 Controls for Identification Codes/Passwords: Outlines measures to ensure the security and integrity of identification codes and passwords, including procedures for their issuance, maintenance, and periodic changing.
Key Components of 21 CFR Part 11 Compliance
Organizations must address several key components, as mentioned below, to ensure compliance with 21 CFR Part 11. Let’s understand these with the help of examples.
System Validation
Organizations must demonstrate that their electronic systems produce accurate, consistent, and reliable results. This process typically involves validating software applications, infrastructure, and hardware systems.
Example: A pharmaceutical company must demonstrate that its laboratory information management system (LIMS), which stores electronic test results, operates consistently as expected under all conditions through rigorous software testing and documentation. Read: how to perform effective Healthcare Software Testing.
Audit Trails
The regulation requires that electronic records include a secure, computer-generated, time-stamped audit trail. This must document the date and time of operator entries and actions that create, modify, or delete electronic records.
Example: An audit trail in a clinical trial data management system that logs every instance of data entry or editing, along with the user’s identity making the change and the exact timestamp of the change. This ensures traceability and accountability in the data handling process.
Data Security and Integrity
Organizations must implement technical and procedural controls to ensure the integrity and confidentiality of electronic records. This includes data encryption, access controls, and backup and recovery processes.
Example: A device manufacturer maintains electronic quality control records in a format that can be readily accessed and reviewed by auditors over the years, using a system that supports data integrity and compatibility with regulatory standards.
Electronic Signatures
To be considered valid, electronic signatures must be unique to the individual, linked to the corresponding electronic record, and require two distinct identification components (such as a user ID and password).
Example: When a scientist approves a batch record in a manufacturing execution system, they must enter their unique username followed by a password or biometric input. It ensures the signature’s validity and non-repudiation.
Record Copies
The ability to generate accurate and complete copies of records in both human-readable and electronic form suitable for inspection, review, and copying by the agency.
Example: A biotech company can produce copies of electronic batch records in PDF format that are easily printable and can be submitted electronically to the FDA during an inspection.
Record Protection
Records must be protected to enable their accurate and ready retrieval throughout the record retention period.
Example: Secure data storage solutions with backup and disaster recovery plans ensure that clinical study data is protected from loss or damage, maintaining integrity and accessibility even in the event of a system failure.
Operational System Checks
Automated procedures that enforce intended use, such as preventing unauthorized access or changes to data and ensuring the sequence of steps in a process.
Example: A software system that manages drug formulation processes automatically checks and confirms that each step in the formulation has been completed correctly before allowing the user to proceed to the next step, thus enforcing procedural compliance.
Training
Employees who use electronic systems must receive appropriate training to ensure they understand the system’s functionality and their responsibilities under 21 CFR Part 11. Train all users on how to use the software in compliance with 21 CFR Part 11. This includes training on the importance of audit trails, proper use of electronic signatures, and security practices.
Implementing 21 CFR Part 11 Compliance
Achieving compliance with 21 CFR Part 11 can be a complex process. Here are some steps to help guide organizations through the process:
- Perform a Gap Analysis: Identify any shortcomings in the organization’s current processes and systems and develop a plan to address these gaps. Ensure that the development, quality assurance, and regulatory teams understand the requirements of 21 CFR Part 11. This includes understanding the specifics around electronic records, electronic signatures, and system security.
- Establish a Cross-Functional Team: Assemble a team of key stakeholders from various departments, including IT, Quality Assurance, and Regulatory Affairs, to oversee the compliance project.
- Develop and Implement Policies and Procedures: Create a comprehensive set of policies and procedures that outline how the organization will achieve and maintain compliance with 21 CFR Part 11.
- Conduct Employee and User Training: Ensure all employees/users who use electronic systems are trained on the new policies, procedures, and system functionality.
- Perform Regular Audits: Regularly assess and audit electronic systems to ensure ongoing compliance and address potential issues. Consider engaging a third party to audit the software and its compliance with 21 CFR Part 11. This can provide validation from an external source and highlight any potential issues.
Certification or seals from recognized industry bodies can enhance the software’s credibility and demonstrate compliance to potential clients.
- Documentation and SOPs: Create comprehensive documentation that covers the development process, validation, system specifications, user guides, and SOPs for using the software. Develop standard operating procedures for creating, modifying, maintaining, and transmitting electronic records. Include guidelines for using electronic signatures.
Achieve 21 CFR Part 11 Compliance with testRigor
Simplify your compliance journey for 21 CFR Part 11 using testRigor for your automated testing needs. Our generative AI-powered system will help you generate screenshots for every screen, test logs, timestamps, username, and error texts, capture video recordings of the test execution, and produce a detailed Word/PDF file at the end. Streamline your compliance requirements with minimal effort and time using these valuable features:
- Use plain English commands to outline test steps: Describe each action and requirement in simple English commands to ensure all stakeholders easily understand the process and participate in the testing process. Team members can write, update, and execute these plain English test cases, irrespective of their programming knowledge.
Even if your team consists of manual testers, they can easily create automation tests with no learning curve using testRigor. Read: All-Inclusive Guide to Test Case Creation in testRigor.
- Capture screenshots of every step executed: With testRigor, you can rest assured knowing that your test steps are being documented in every way possible.
- Compile a comprehensive Word/PDF/CSV report: Consolidate your compliance documentation, including screenshots, error texts, timestamps, logs, and actions taken at each step, into a single Word/PDF file for easy reference and submission to regulatory authorities. Easily export the test suite details in CSV files.
- Cover complex scenarios with simplicity: Develop a comprehensive test plan that outlines scenarios specifically for 21 CFR Part 11 requirements. For instance, tests for user authentication, authorization, electronic signatures, audit trails, and system security features. testRigor helps you validate files, audio, 2FA, video, email, SMS, phone calls, mathematical validations/calculations of formulas, QR codes, database testing, Captcha resolution, APIs, Chrome extensions, data-driven testing, form filling, AI features, and many more complex scenarios.
- Effortless end-to-end and functional testing: testRigor is the best companion for performing quick end-to-end or functional test cases in plain English. Moreover, you can also import/ copy your manual test cases from all test management tools such as TestRail, Qase, PractiTest, etc.
Read this step-by-step guide to understand How to do End-to-end Testing with testRigor.
- Powerful and seamless integrations: testRigor integrates with all significant test management, infrastructure providers, ticketing systems, ERP, CRM, CI/CD tools. Build your testing ecosystem using plain English commands and these simple integrations.
- Test the Untestable: Use testRigor’s other AI-based features that help test the untestable, like graphs, images, chatbots, LLMs, Flutter apps, and many more.
How to View testRigor’s 21 CFR Part 11 Compliant Test Results?
You can view these 21 CFR Part 11 compliant test results from a couple of places within testRigor.
1. See individual, step-by-step test runs for every test case
You can glance through all the test cases in a test suite, along with each of their latest test runs and detailed results under the Test Cases section.
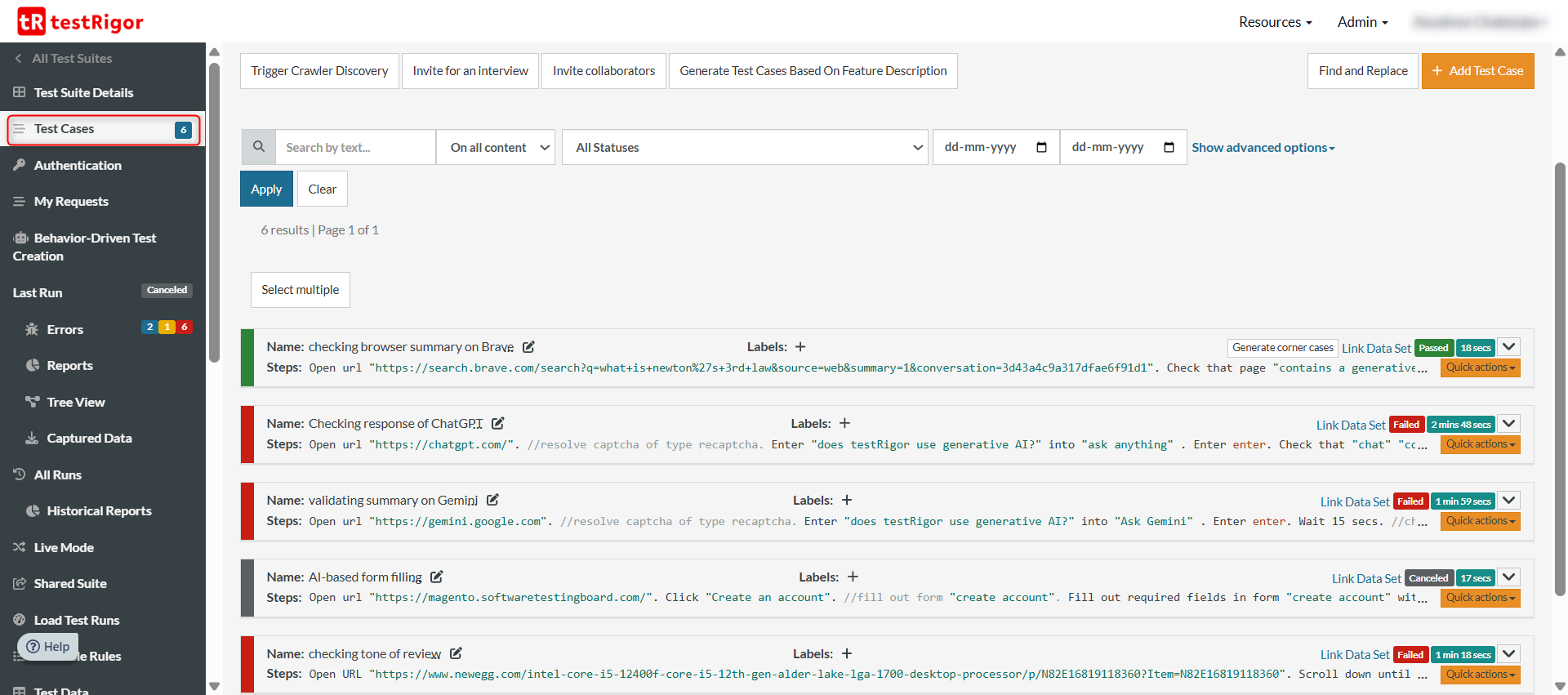
2. See the results of a test suite run
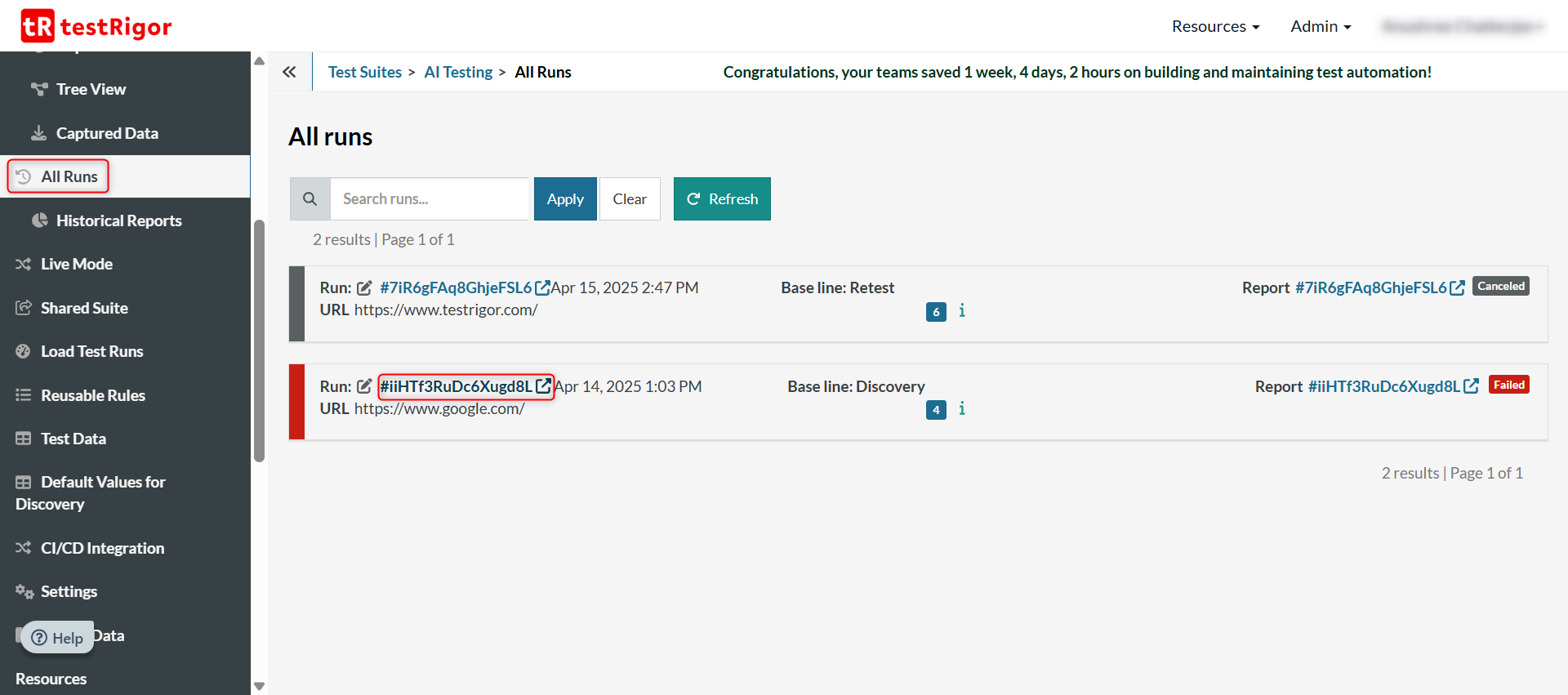
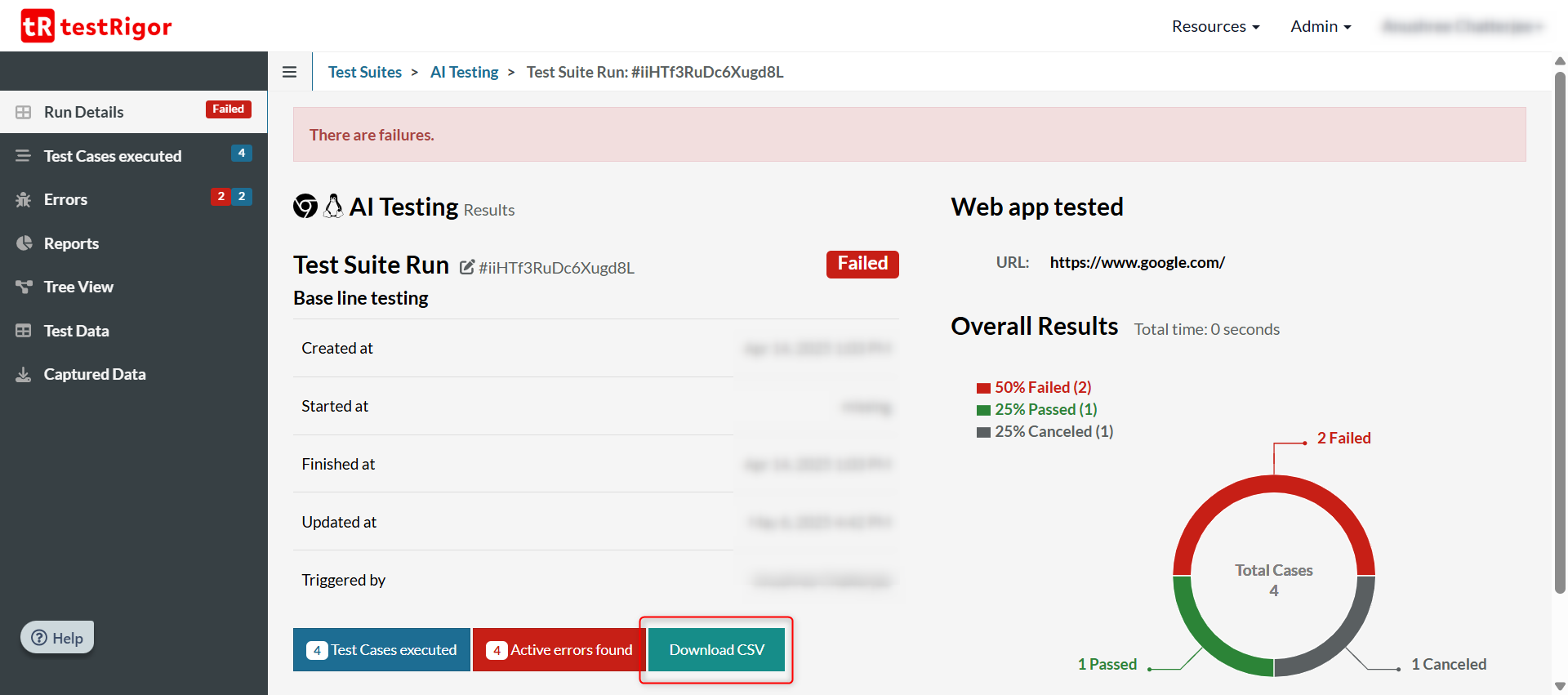
You can view the results of the latest run of the test suite too which may not include all the test cases. For that, click on All Runs in the left menu.
Here, you’ll see an overview of what was executed. To get a more detailed view, click on the link of any test run in this list.
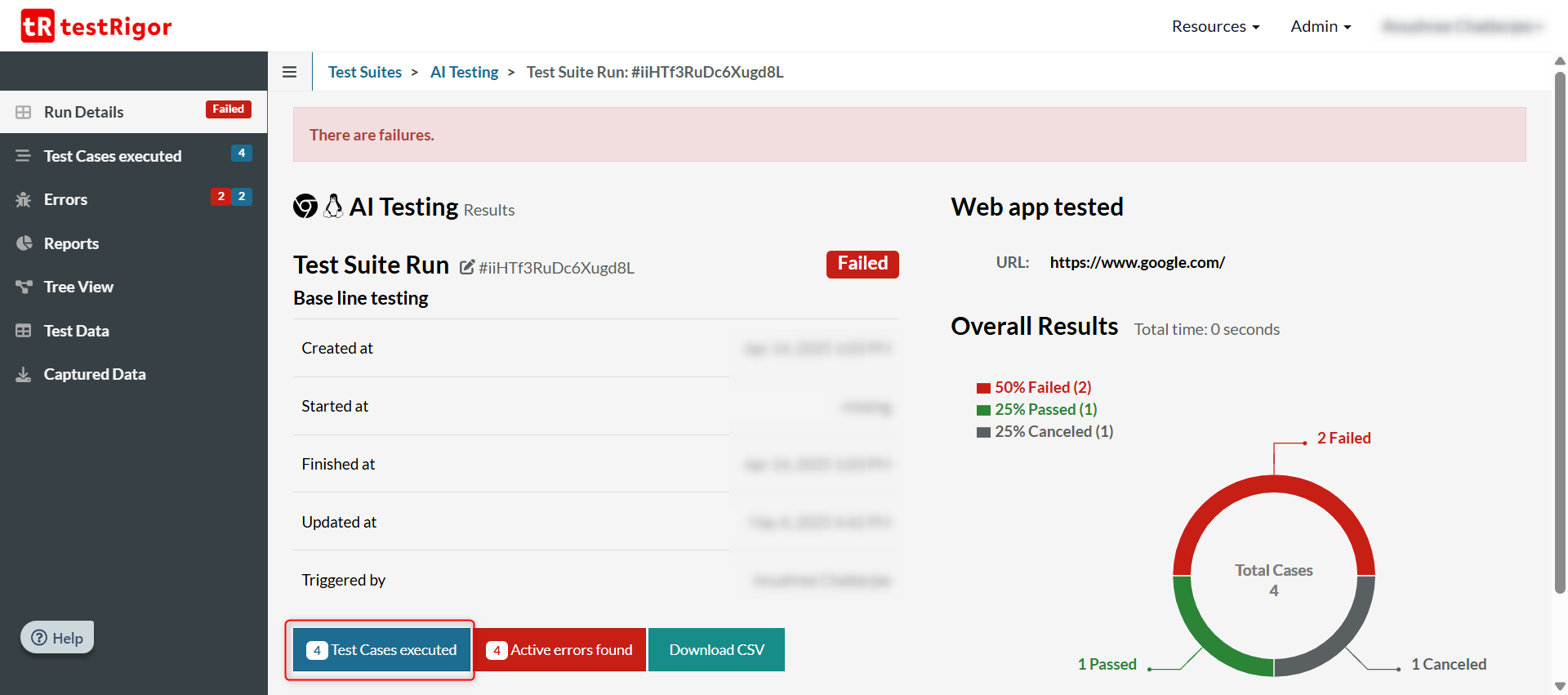
Click on Test Cases Executed to see all the test cases that were executed in the last run.
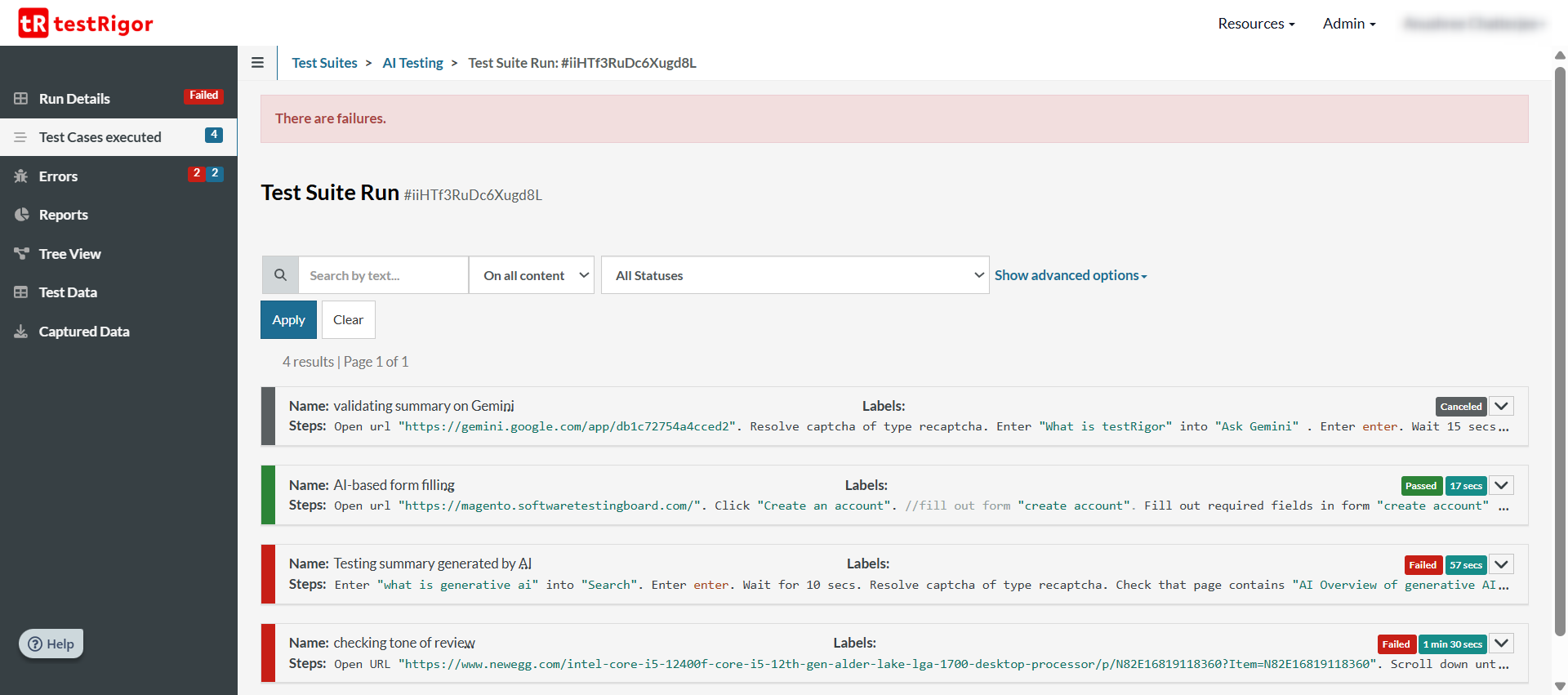
With each test case, you’ll see a step-by-step documentation of the test steps along with screenshots.
3. Generate a detailed PDF or Word document of the test execution
To view the execution results, navigate to the Test Cases tab and click on View execution.
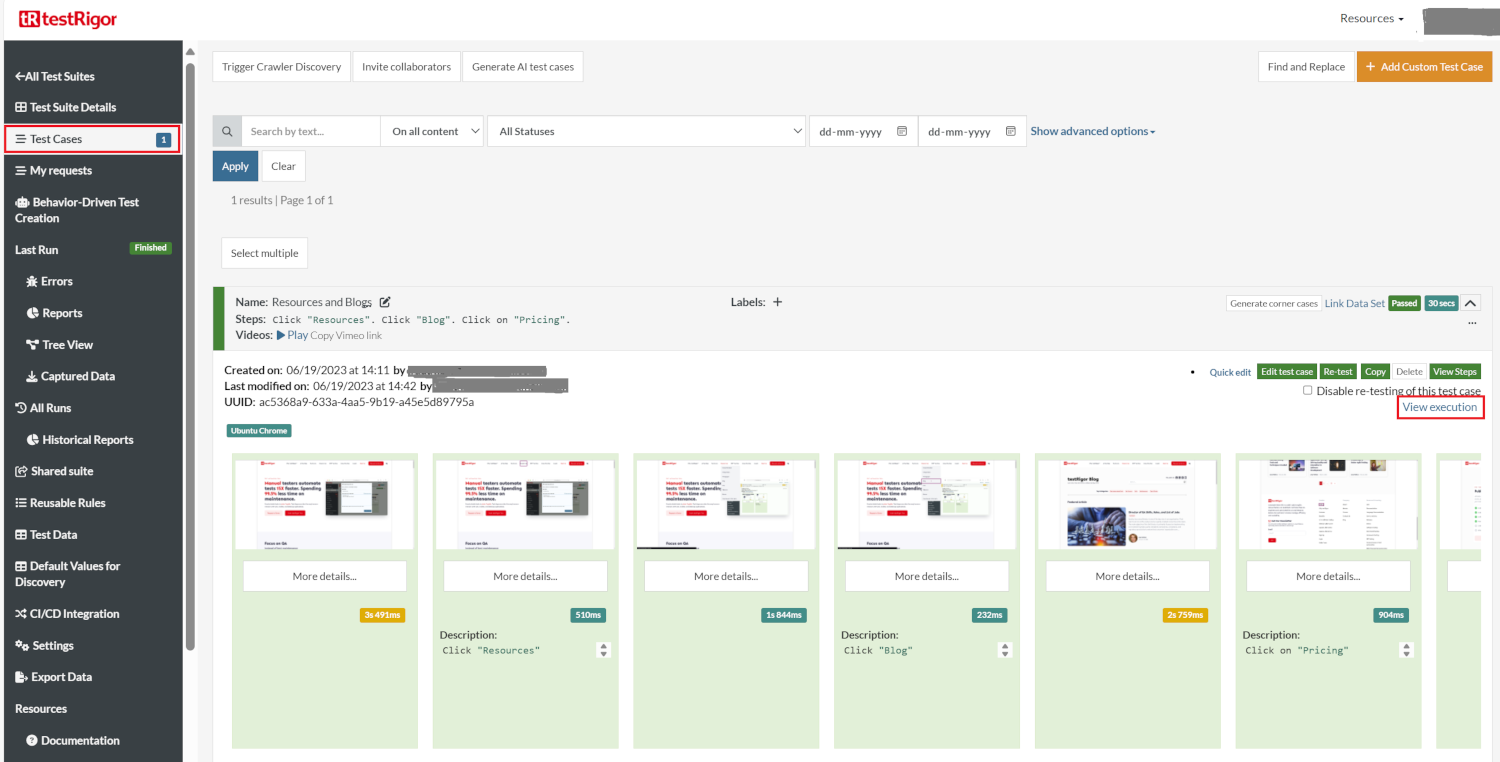
In the execution results, you can ‘Generate PDF‘ or ‘Download Word‘ files with screenshots.
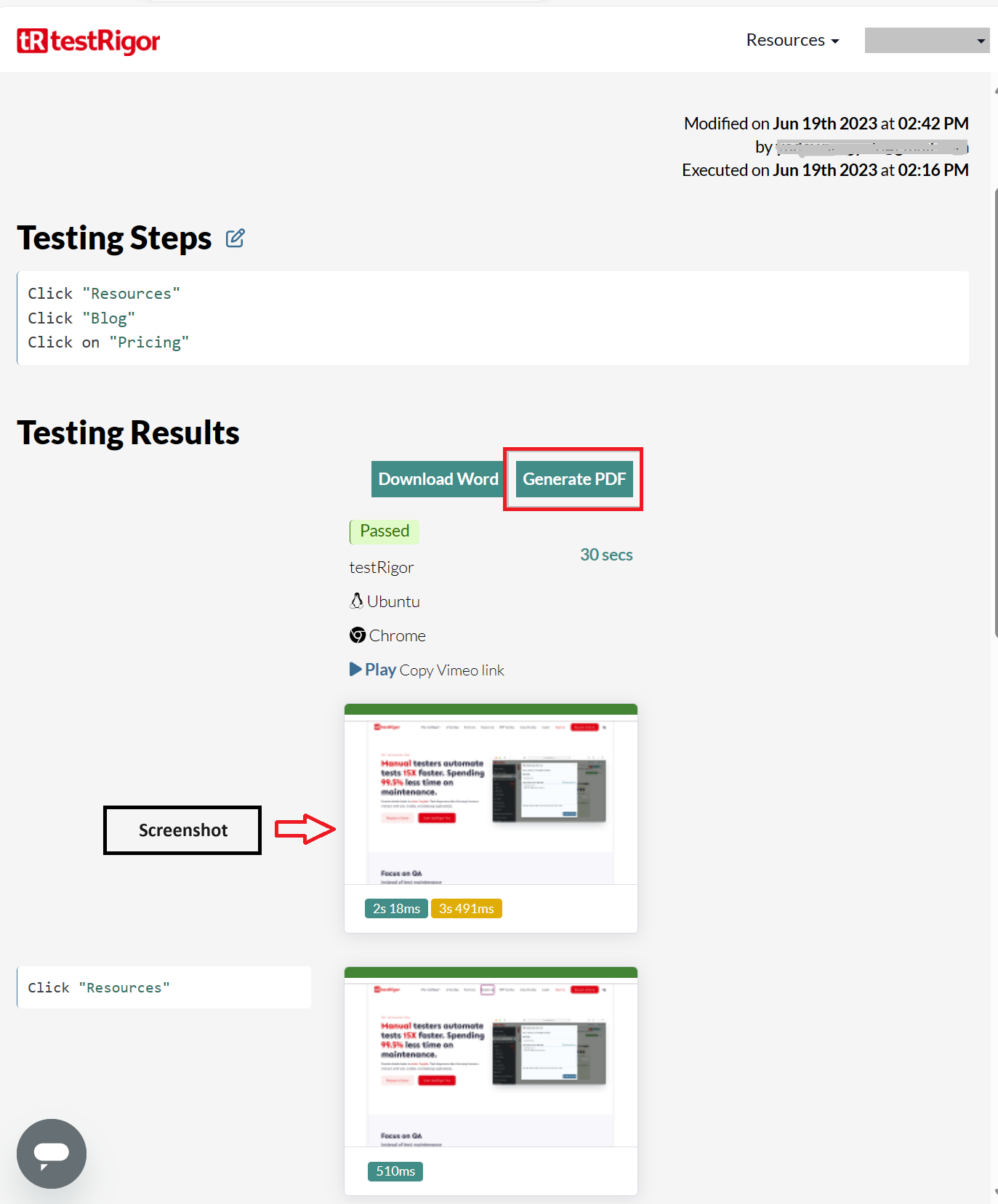
Here is a sample PDF report, compliant with 21 CFR Part 11:

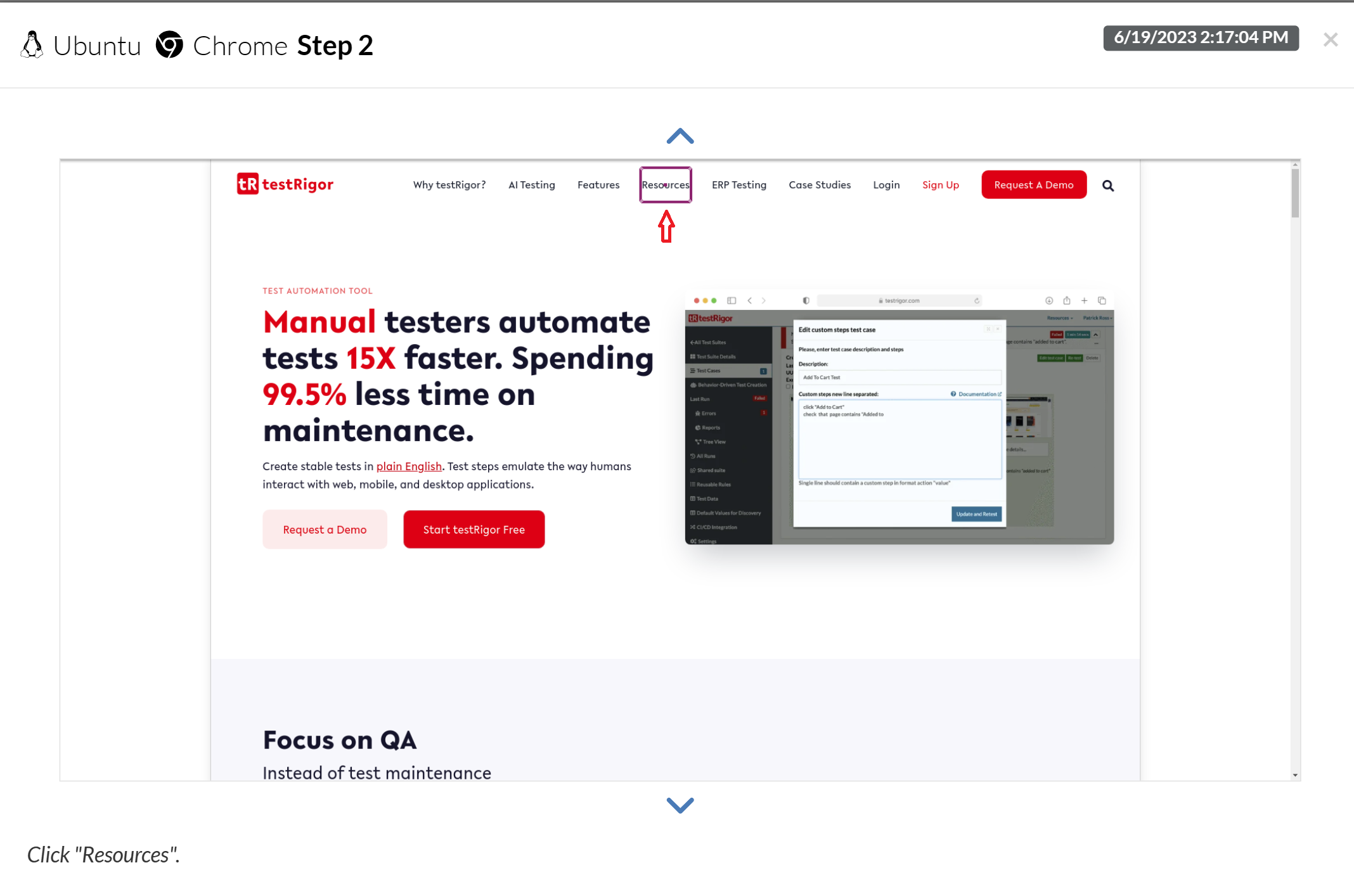
This is a screenshot of the test execution for one test step:
4. Download the CSV document with test suite details
You can export the test suite details, including the test cases and their runs in the suite, along with their URLs.
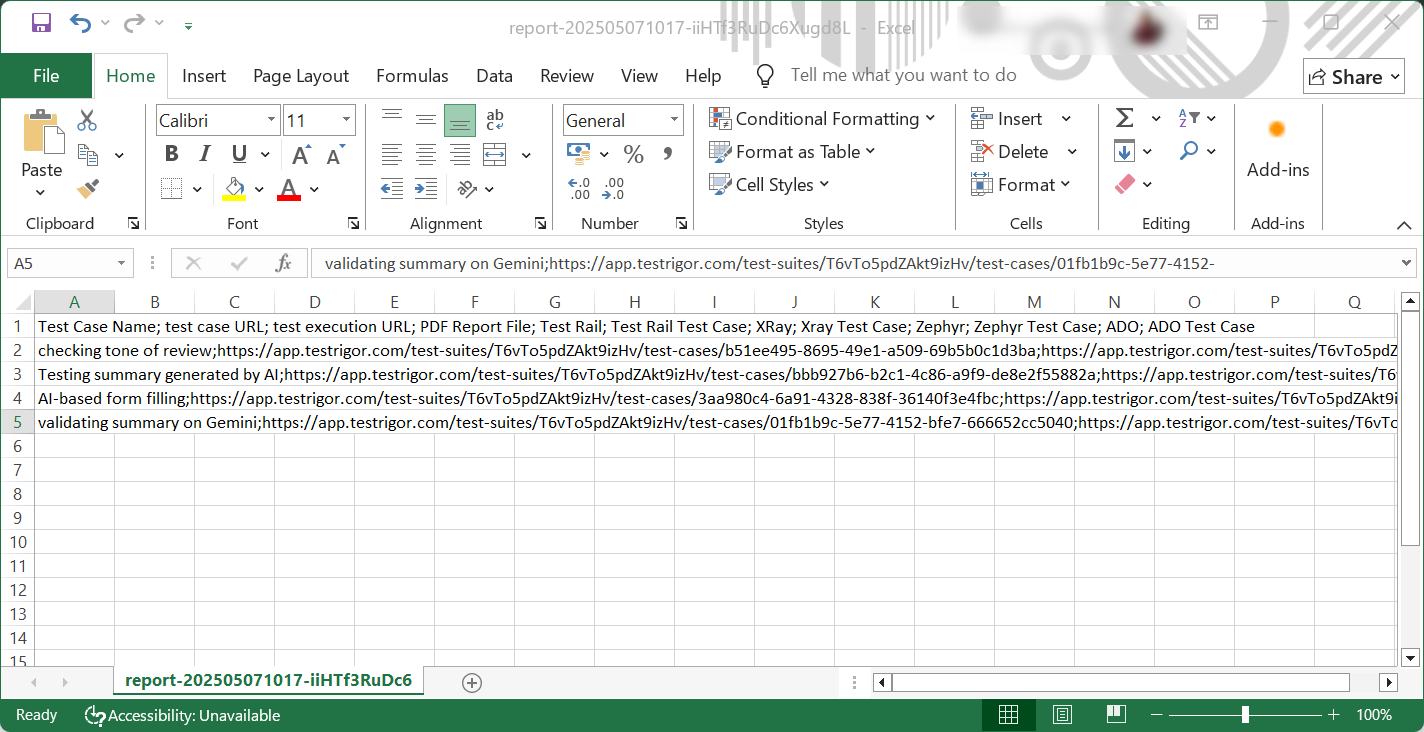
To do this, you need to click on All Runs in the left menu. Then click on the link of the test run of a non-cancelled test case.
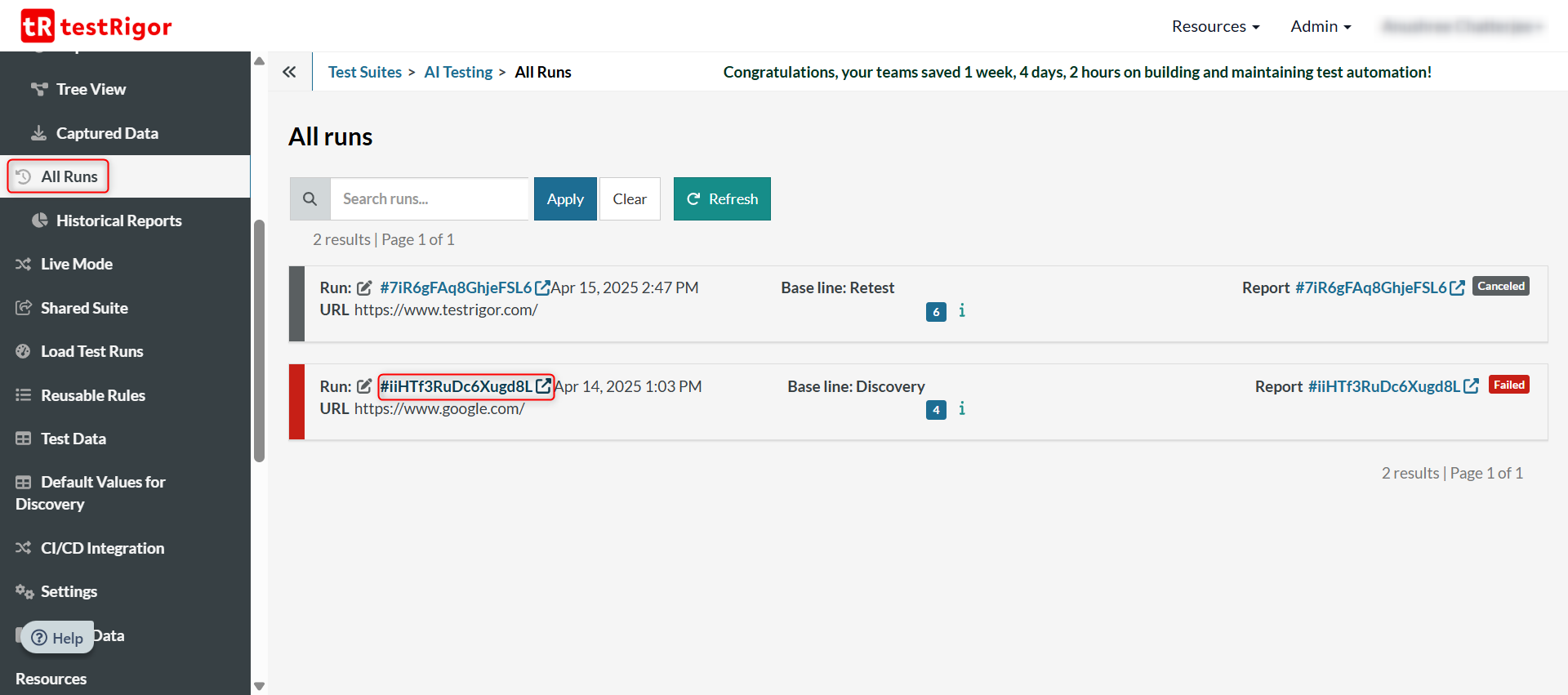
Click on the Download CSV option to get the document.
Using testRigor, you can perform cross-browser and cross-platform testing single-handedly. Execute test cases on the web, mobile (native, hybrid), desktop, mainframes, and API using plain English commands.
Access testRigor documentation and top testRigor’s features to learn about more valuable capabilities.
Conclusion
Incorporating 21 CFR Part 11 compliance is crucial for organizations operating within FDA-regulated industries that utilize electronic records and signatures. Intelligent AI agents like testRigor can automate and streamline the compliance process, ensuring accuracy, consistency, and reliability.
testRigor is SOC2 and HIPAA compliant and supports FDA 21 CFR Part 11 reporting. You can efficiently perform accessibility testing through testRigor. Read here how to build an ADA-compliant app.
testRigor’s powerful capabilities, combined with the guidance provided in this article, enable organizations to effortlessly navigate the complex requirements of 21 CFR Part 11 and maintain long-term compliance. Embracing such technologies supports regulatory approval and reinforces data security and integrity, ultimately benefiting the whole organization and its stakeholders.
| Achieve More Than 90% Test Automation | |
| Step by Step Walkthroughs and Help | |
| 14 Day Free Trial, Cancel Anytime |












